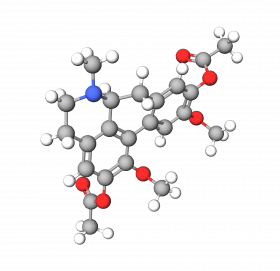
Diacetyl Boldine (DAB) is a derivative of Boldine, an alkaloid initially found in the Boldo (Peumus boldus). It is also known under the Sederma's trade name Lumiskin™.
Diacetyl Boldine stabilizes Tyrosinase in its inactive form. Tyrosinase is one of the critical enzymes involved in melanin production, so preventing its activation decreased pigment production, leaving a skin-brightening effect. In addition, DAB directly inhibits Tyrosinase, resulting in about a 53% drop in enzyme activity.
Thanks to its dual action (inactivation and inhibition), Diacetyl Boldine reduces melanin production by up to 70%, revealing a brighter, more uniform, and more radiant complexion.
In scientific studies using a skin model with DAB-treated cells was found to display a visible and significant reduction in epidermal pigmentation. Topical application of Diacetyl Boldine has been suggested as a chemoprotection treatment against Melanoma.In vivo colorimetric studies with a preparation containing Diacetyl Boldine confirmed a lower melanin index and a higher Individual Typology Angle (ITA, calculated from digital images using specific algorithms). During the self-evaluation of the tests, most panelists noted a decrease in pigmentation, age, and dark spots.