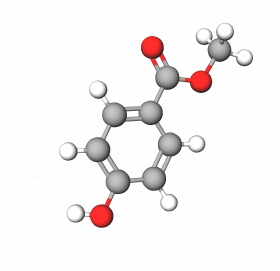
Methylparaben is a methyl ester of p-hydroxybenzoic acid, a widely used broad-spectrum preservative used in personal care products, pharmaceuticals, and some foods. It is colorless and odorless, compatible with a wide range of cosmetic ingredients.
Methylparaben and other members of the parabens family are relatively effective compared to other organic acid-based preservatives, thanks to their stable efficacy across a wide pH range and other conditions. Another notable property of parabens is that their antimicrobial activity covers practically all the microorganisms responsible for spoiling cosmetic and pharmaceutical products.
Methylparaben is a standard preservative for cosmetics and pharmaceuticals, with a slightly acidic to neutral pH that cannot generally be altered. Because its effect is independent of the acidity of the product to be protected, it is particularly reliable in cases where, owing to an error, the pH of the substance is not adjusted to the correct level.
If used in recommended concentrations, Methylparaben has a low order of toxicity and ecotoxicity. It exhibits a broad, balanced spectrum of antimicrobial activity against germs, retaining efficacy over a pH range of 4 to 8. In combination with other parabens, Methylparaben potentiates their action and increases activity, allowing formulators to lower concentrations (lower minimum inhibitory concentration).Methylparaben is a preservative of medium strength with generally adequate water solubility. It is indispensable for preserving many cosmetics and pharmaceuticals. A combination of Methylparaben and Propylparaben is often the best solution.
There were many discussions and manipulations about the harmful or adverse effects of Methylparaben and other parabens. However, it was found to be non-mutagenic (including chromosomal aberrations), non-carcinogenic, non-teratogenic, non-spermatotoxic, non-toxic, and rarely irritating or sensitizing at concentrations used in cosmetics.
An interesting study reveals the mechanism of Methylparaben's sensory irritation in sensitive skin. In normal skin, it almost instantly hydrolizes into PABA, which doesn't irritate. The responsible enzyme is Carboxylesterase 1. In sensitive skin, hydrolisation is slower, and relatively higher amounts of remaining Methylparaben can irritate. So, sensitivity may be related to MP's sluggish metabolism.